
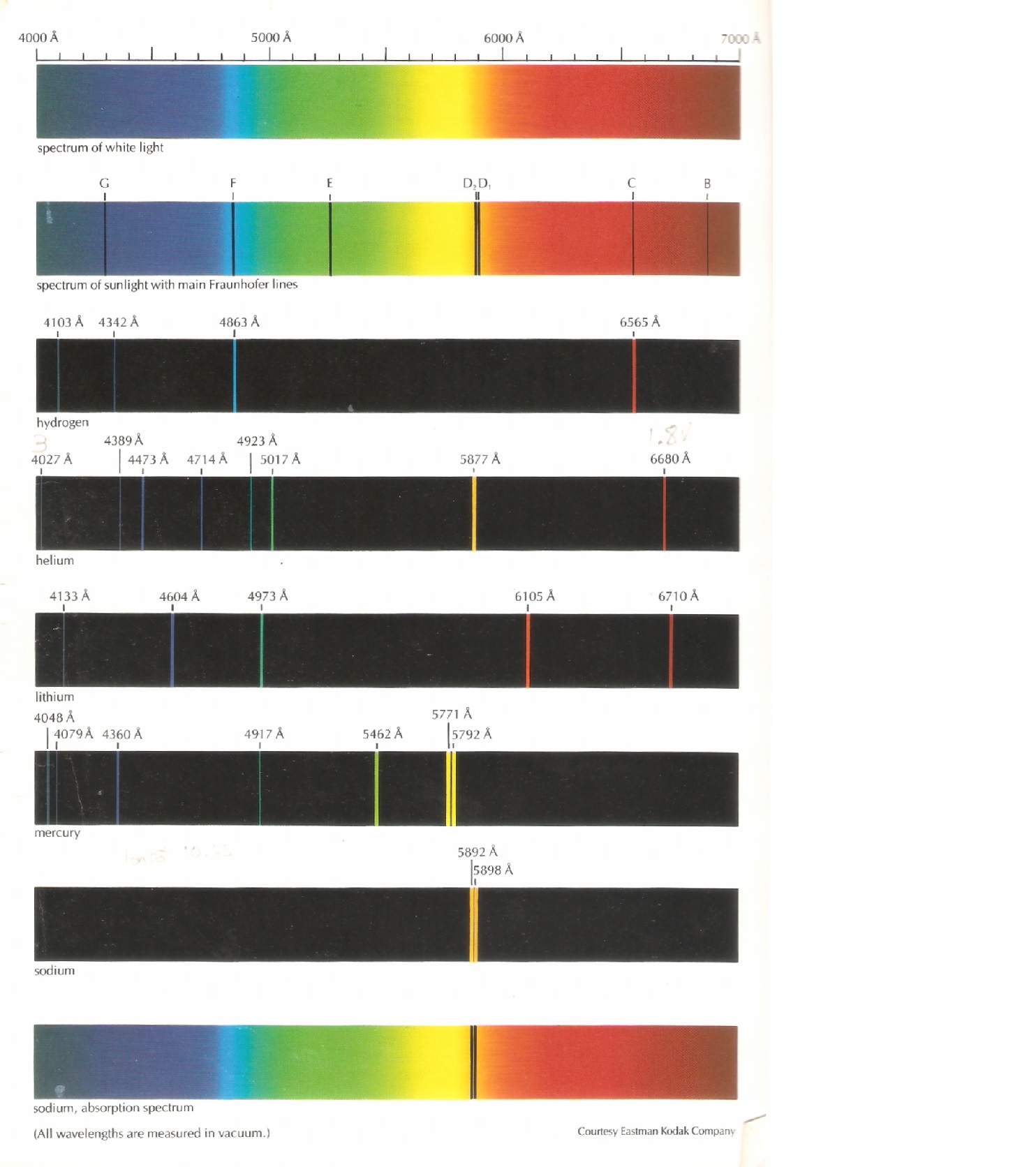
Cambridge, MA: Harvard University Press, 1963. New York, NY: Cambridge University Press, 1996. Cecilia Payne-Gaposchkin: An Autobiography and Other Recollections. This lecture gives more details about the atomic spectra of hydrogen along with matter/energy interactions involving atomic hydrogen. Lecture Slides (PDF - 1.3MB) Lecture Summary Explain how emission line spectra and absorption line spectra are formed. Explanation: When an atom absorbs energy, its electrons jump to higher energy levels. You can look at the spectra and identify which elements are present.

Line spectra the Bohr model uses of emission and absorption spectra Each element has its own unique atomic emission spectrum. For Hydrogen, we mainly see the Balmer series, with a line from. 6.3, “Atomic Spectra and Models of the Atom.” The figure below shows the visible part of the spectrum for several atomic or molecular sources. What you see is determined by environment. How does Bohrs atomic theory explain the unique bright-line spectra of the elements The farther an electron is from the nucleus, the more energy that. The setup involves using an STM tip to measure a tunneling current (red beam) induced by the illumination of a sample with x rays (blue beam). Absorption and emission spectral lines are at exactly the same wavelengths.

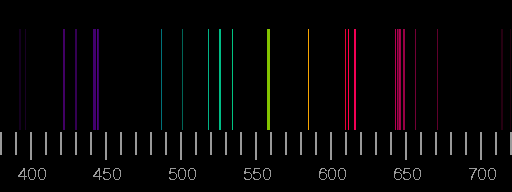
The following diagram represents some electronic transitions in a hydrogen atom. Sadoway discusses the shell model and quantum numbers ( Session 5). Practice: Emission and Absorption Spectra. Session 3: Atomic Models: Rutherford & Bohr An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies since the re-emitted light.Place the helium spectral tube in the holder and adjust its position for maximum intensity when viewed through the slit from the front. Matter/Energy Interactions: Atomic SpectraĪtomic spectra of hydrogen, matter/energy interactions involving atomic hydrogen, planetary model, Bohr’s postulates, quantum condition, ionization energy, electron orbital transitionsĪngstrom, Avogadro’s number, prism, refraction, wavelength, nanometer, Johann Balmer, wavenumber, Michael Faraday, cathode, anode, electron-volt, Bohr radius, ground state, ionization energy, energy level, conservation of energy, atomic spectra, Cecilia Payne, Ernest Rutherford, joule, coulomb, Max Planck, Planck’s constant, emission spectra, spectrograph, electrode, photon, volt, radiationĬhemical analysis, analyzing composition of stars, televisionīefore starting this session, you should be familiar with: Place the diffraction grating in the mount at the center of the round metal diffractometer table and align it perpendicular to the path of the light. When exploding fireworks, you will see the color when the metal atoms absorb energy from the detonater.Structure of the Atom 4.(CC BY-NC-SA 3.0 Christopher Auyeung via CK-12 Foundation) So if we can see a spectral line at a given wavelength, it is the result of an electron emitting light as it drops to a lower energy level.


 0 kommentar(er)
0 kommentar(er)
